
 N2(g)+
N2(g)+ H2(g) = NH3(l) ;△H.= (b+c—a) kJ/mol
H2(g) = NH3(l) ;△H.= (b+c—a) kJ/mol  N2(g)+
N2(g)+ H2(g) =NH3(g) ;△H.= (a+b) kJ/mol
H2(g) =NH3(g) ;△H.= (a+b) kJ/mol
 H2的燃烧热为241.8 kJ·mol-1 由反应②知在温度一定的条件下,在一恒容密闭容器中通入1 mol N2和3 mol H2,反应后放出的热量为Q1 kJ,若通入2 mol N2和6 mol H2充分反应后放出的热量为Q2 kJ,则184.8>Q2>2Q1 氨的催化氧化反应为4NH3(g)+5O2(g)=4NO(g)+6H2O(g) ΔH.=+906 kJ·mol-1
H2的燃烧热为241.8 kJ·mol-1 由反应②知在温度一定的条件下,在一恒容密闭容器中通入1 mol N2和3 mol H2,反应后放出的热量为Q1 kJ,若通入2 mol N2和6 mol H2充分反应后放出的热量为Q2 kJ,则184.8>Q2>2Q1 氨的催化氧化反应为4NH3(g)+5O2(g)=4NO(g)+6H2O(g) ΔH.=+906 kJ·mol-1
 2NH3(g)ΔH.=-Q.1 kJ/mol(Q.1>0) H2(g)+CO(g)
2NH3(g)ΔH.=-Q.1 kJ/mol(Q.1>0) H2(g)+CO(g)  (s)+H2O(g)ΔH.=+Q.4 kJ/mol(Q.4>0) C.4NH3(g)+5O2(g)
(s)+H2O(g)ΔH.=+Q.4 kJ/mol(Q.4>0) C.4NH3(g)+5O2(g)  4NO(g)+6H2O(g)ΔH.=-Q.3 kJ/mol(Q.3>0) 2SO3(g)
4NO(g)+6H2O(g)ΔH.=-Q.3 kJ/mol(Q.3>0) 2SO3(g) 2SO2(g)+O2(g)ΔH.=+Q.2 kJ/mol(Q.2>0)
2SO2(g)+O2(g)ΔH.=+Q.2 kJ/mol(Q.2>0)
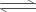 2NH3(g) △H=―Q1kJ・mol―1(Q1>0) 2SO3(g)
2NH3(g) △H=―Q1kJ・mol―1(Q1>0) 2SO3(g) 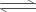 2SO2(g)+O2(g) △H=+Q2kJ・mol―1(Q2>0) 4NH3(g)+5O2(g)
2SO2(g)+O2(g) △H=+Q2kJ・mol―1(Q2>0) 4NH3(g)+5O2(g)  4NO(g)+6H2O(g) △H=―Q3kJ・mol―1(Q3>0) H2(g) +CO(g)
4NO(g)+6H2O(g) △H=―Q3kJ・mol―1(Q3>0) H2(g) +CO(g)  C.(s)+H2O(g) △H=+Q4kJ・mol―1(Q4>0)
C.(s)+H2O(g) △H=+Q4kJ・mol―1(Q4>0)
 2NH3(g) ΔH.=-Q.1 kJ·mol-1(Q.1>0) 2SO3(g)
2NH3(g) ΔH.=-Q.1 kJ·mol-1(Q.1>0) 2SO3(g) 2SO2(g)+O2(g) ΔH.=+Q.2 kJ·mol-1(Q.2>0) 4NH3(g)+5O2(g)
2SO2(g)+O2(g) ΔH.=+Q.2 kJ·mol-1(Q.2>0) 4NH3(g)+5O2(g) 4NO(g)+6H2O(g) ΔH.=-Q.3 kJ·mol-1(Q.3>0) H2(g)+CO(g)
4NO(g)+6H2O(g) ΔH.=-Q.3 kJ·mol-1(Q.3>0) H2(g)+CO(g) C.(s)+H2O(g) ΔH.=+Q.4 kJ·mol-1(Q.4>0)
C.(s)+H2O(g) ΔH.=+Q.4 kJ·mol-1(Q.4>0)
 2NH3(l) ΔH.=-Q kJ/mol,则Q.>92.4 在密闭容器中加入1 mol N2和3 mol H2充分反应放热92.4 kJ 增大压强,平衡向右移动,平衡常数增大 若一定条件下反应达到平衡,N2的转化率为20%,则H2的转化率一定为60%
2NH3(l) ΔH.=-Q kJ/mol,则Q.>92.4 在密闭容器中加入1 mol N2和3 mol H2充分反应放热92.4 kJ 增大压强,平衡向右移动,平衡常数增大 若一定条件下反应达到平衡,N2的转化率为20%,则H2的转化率一定为60%
 2NH3(g)ΔH<0达到平衡后,升高温度,反 应速率v(H2)和H2的平衡转化率均增大 已知氯气、溴蒸气分别跟氢气反应的热化学方程式如下(Q1、Q2的值 均大于零): H2(g) +Cl2(g)=2HCl(g) △H1=—Q1 kJ/mol H2(g) +Br2(g)=2HBr(g) △H2=—Q2 kJ/mol 则△H1<△H2
2NH3(g)ΔH<0达到平衡后,升高温度,反 应速率v(H2)和H2的平衡转化率均增大 已知氯气、溴蒸气分别跟氢气反应的热化学方程式如下(Q1、Q2的值 均大于零): H2(g) +Cl2(g)=2HCl(g) △H1=—Q1 kJ/mol H2(g) +Br2(g)=2HBr(g) △H2=—Q2 kJ/mol 则△H1<△H2

 2NH3(g)ΔH.=-Q.1 kJ/mol(Q.1>0) H2(g)+CO(g)
2NH3(g)ΔH.=-Q.1 kJ/mol(Q.1>0) H2(g)+CO(g)  (s)+H2O(g)ΔH.=+Q.4 kJ/mol(Q.4>0) C.4NH3(g)+5O2(g)
(s)+H2O(g)ΔH.=+Q.4 kJ/mol(Q.4>0) C.4NH3(g)+5O2(g)  4NO(g)+6H2O(g)ΔH.=-Q.3 kJ/mol(Q.3>0) 2SO3(g)
4NO(g)+6H2O(g)ΔH.=-Q.3 kJ/mol(Q.3>0) 2SO3(g) 2SO2(g)+O2(g)ΔH.=+Q.2 kJ/mol(Q.2>0)
2SO2(g)+O2(g)ΔH.=+Q.2 kJ/mol(Q.2>0)