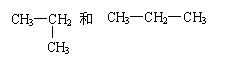你可能感兴趣的试题
HOCH2COOCH2CH3 CH3CH(OH)CH2COOH HOCH2CH2CH2COOH CH3CH2CH(OH)COOH
每有0.1mol O2反应,则迁移H+ 0.4mol 正极反应式为:CH2=CH2-2e- + 2OH- = CH3CHO + H2O 电子移动方向:电极a→磷酸溶液→电极b 该电池为可充电电池
CH3OH(l)+ O2(g)===CO2(g)+2H2O(l) ΔH =+725.8 kJ/mol 2CH3OH(l)+3O2(g)===2CO2(g)+4H2O(l) ΔH =-1452 kJ/mol 2CH3OH(l)+3O2(g)===2CO2(g)+4H2O (l) ΔH =-725.8 kJ/mol 2CH3OH(l)+3O2(g)===2CO2(g)+4H2O(l) ΔH =+1452 kJ/mol
O2(g)===CO2(g)+2H2O(l) ΔH =+725.8 kJ/mol 2CH3OH(l)+3O2(g)===2CO2(g)+4H2O(l) ΔH =-1452 kJ/mol 2CH3OH(l)+3O2(g)===2CO2(g)+4H2O (l) ΔH =-725.8 kJ/mol 2CH3OH(l)+3O2(g)===2CO2(g)+4H2O(l) ΔH =+1452 kJ/mol
CH3OH的燃烧热为192.9 kJ/mol 反应①中的能量变化如下图所示 CH3OH转变成H2的过程一定要吸收能量 根据②推知反应CH3OH(l)+ O2(g)===CO2(g)+2H2(g)的ΔH>-192.9 kJ/mol
O2(g)===CO2(g)+2H2(g)的ΔH>-192.9 kJ/mol
CH4与C.2H4 与 CH3-CH2-CH2-CH3 C.2H6与C.3H8 O2与O3
CH3OH(l)+ O2(g)===CO2(g)+2H2O(l);ΔH=+725.8 kJ/mol 2CH3OH(l)+3O2(g)===2CO2(g)+4H2O(l);ΔH=-1452 kJ/mol 2CH3OH(l)+3O2(g)===2CO2(g)+4H2O(l);ΔH=-725.8 kJ/mol 2CH3OH(l)+3O2(g)===2CO2(g)+4H2O(l);ΔH=+1452 kJ/mol
O2(g)===CO2(g)+2H2O(l);ΔH=+725.8 kJ/mol 2CH3OH(l)+3O2(g)===2CO2(g)+4H2O(l);ΔH=-1452 kJ/mol 2CH3OH(l)+3O2(g)===2CO2(g)+4H2O(l);ΔH=-725.8 kJ/mol 2CH3OH(l)+3O2(g)===2CO2(g)+4H2O(l);ΔH=+1452 kJ/mol
CH3OH CH3CH2OH HOCH2CH2OH HCHO
HOCH2COOCH2CH3
CH3CH(OH)CH2COOH
HOCH2CH2CH2COOH
CH3CH2CH(OH)COOH
O2 和O3 CH2=CHCH2CH3 和CH3CH=CHCH3 CH3CH2OH 和CH3OCH3 CH3CH2CH3 和CH3(CH2)2CH3
CH3COOH + OH- CH3COO-+H2O S2-+2H2O H2S+2OH- CH3COOH+H2O CH3COO-+H3O+ NH4++H2O NH3• H2O+H+
每有0.1mol O2反应,则迁移H+ 0.4mol 正极反应式为:CH2=CH2-2e- + 2OH- = CH3CHO + H2O 电子移动方向:电极a→磷酸溶液→电极b 该电池为可充电电池
CH3—O.—CH3和CH3—CH2—OH CH4 和CH3CH3 O2 和O3 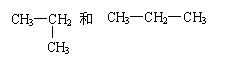
CH4和CH3CH3 CH3CH2OH和CH3OCH3 NO2和N2O4 O2和O3
O2和O3 CH2=CHCH2CH3和CH3CH=CHCH3 CH3CH2OH和CH3OCH3 CH3CH2CH3和CH3(CH2)2CH3
O2和O3 16O.和18O. CH3CH2OH和CH3OCH3 CH3CH2CH3和CH3(CH2)2CH3

 O2(g)===CO2(g)+2H2O(l) ΔH =+725.8 kJ/mol 2CH3OH(l)+3O2(g)===2CO2(g)+4H2O(l) ΔH =-1452 kJ/mol 2CH3OH(l)+3O2(g)===2CO2(g)+4H2O (l) ΔH =-725.8 kJ/mol 2CH3OH(l)+3O2(g)===2CO2(g)+4H2O(l) ΔH =+1452 kJ/mol
O2(g)===CO2(g)+2H2O(l) ΔH =+725.8 kJ/mol 2CH3OH(l)+3O2(g)===2CO2(g)+4H2O(l) ΔH =-1452 kJ/mol 2CH3OH(l)+3O2(g)===2CO2(g)+4H2O (l) ΔH =-725.8 kJ/mol 2CH3OH(l)+3O2(g)===2CO2(g)+4H2O(l) ΔH =+1452 kJ/mol
 O2(g)===CO2(g)+2H2(g)的ΔH>-192.9 kJ/mol
O2(g)===CO2(g)+2H2(g)的ΔH>-192.9 kJ/mol
 O2(g)===CO2(g)+2H2O(l);ΔH=+725.8 kJ/mol 2CH3OH(l)+3O2(g)===2CO2(g)+4H2O(l);ΔH=-1452 kJ/mol 2CH3OH(l)+3O2(g)===2CO2(g)+4H2O(l);ΔH=-725.8 kJ/mol 2CH3OH(l)+3O2(g)===2CO2(g)+4H2O(l);ΔH=+1452 kJ/mol
O2(g)===CO2(g)+2H2O(l);ΔH=+725.8 kJ/mol 2CH3OH(l)+3O2(g)===2CO2(g)+4H2O(l);ΔH=-1452 kJ/mol 2CH3OH(l)+3O2(g)===2CO2(g)+4H2O(l);ΔH=-725.8 kJ/mol 2CH3OH(l)+3O2(g)===2CO2(g)+4H2O(l);ΔH=+1452 kJ/mol