你可能感兴趣的试题
CO的燃烧热为283 kJ 右图可表示由CO生成CO2的反应过程和能量关系 2Na2O2(s)+2CO2(s)=2Na2CO3(s)+O2(g) △H= - 452 kJ/mol CO(g)与Na2O2(s)反应放出509 kJ热量时,电子转移数为2x6.02×l023
C(s)+O2(g)→CO2(g) 2SO2(g)+O2(g)→2SO3(g) 3H2(g)+N2(g)→2NH3(g) CuSO4(s)+5H2O(l)→CuSO4•5H2O(s)
-82kJ/mol -41kJ/mol -312kJ/mol +82kJ/mol
ΔH1>0,ΔH3<0 ΔH2>0,ΔH4>0 ΔH1=ΔH2+ΔH3 ΔH3=ΔH4+ΔH5
CO的燃烧热为283 kJ 右图可表示由CO生成CO2的反应过程和能量关系 2Na2O2(s)+2CO2(s)=2Na2CO3(s)+O2(g) ΔH.>-452 kJ/mol CO(g)与Na2O2(s)反应放出509 kJ热量时,电子转移数为6.02×1023
C(s)+O2(g)=CO2(g) C(s)+O2(g)=CO2(g), (298.15K)=-393.51kJ/mol 2C(s)+2O2(g)=2CO2(g),
(298.15K)=-393.51kJ/mol 2C(s)+2O2(g)=2CO2(g), (298.15K)=-393.51kJ/mol C+O2=CO2,
(298.15K)=-393.51kJ/mol C+O2=CO2, (298.15K)=-393.51kJ/mol
(298.15K)=-393.51kJ/mol
C(s)+O2(g)=CO2(g) C(s)+O2(g)=CO2(g), 2C(s)+2O2(g)=2CO2(g),
2C(s)+2O2(g)=2CO2(g), C+O2=CO2,
C+O2=CO2,
-283.01 kJ·mol-1 +172.51 kJ·mol-1 +283.1 kJ·mol-1 +504.00 kJ·mol-1
2H2(g) + O2(g)= 2H2O(g) △H1 ; 2H2(g) + O2(g) = 2H2O(l) △H2 S(g) + O2(g) =SO2(g) △H1 ; S(s) + O2(g) = SO2(g) △H2 CO(g) + 1/2 O2(g) = CO2(g) △H1 ;2CO(g) + O2(g) = 2CO2(g) △H2 C.(s)+H2O(g)=CO(g)+H2(g) △H1 ;CaO(s)+H2O(l)===Ca(OH)2(s) △H2
C.(s) + 1/2 O2(g) = CO(g) △H= -110.5 kJ/mol 2H2(g) + O2(g) = 2H2O(l) △H= -571.6kJ/mol CH4(g) + 2O2(g) = CO2(g) + 2H2O(g) △H= -802.3kJ/mol CO(g) + 1/2 O2(g) = CO2(g) △H= -283.0kJ/mol
283. kJ·mol-1 +172.5 kJ·mol-1 -172.5 kJ·mol-1l -504 kJ·mol-1

 (298.15K)=-393.51kJ/mol 2C(s)+2O2(g)=2CO2(g),
(298.15K)=-393.51kJ/mol 2C(s)+2O2(g)=2CO2(g), (298.15K)=-393.51kJ/mol C+O2=CO2,
(298.15K)=-393.51kJ/mol C+O2=CO2, (298.15K)=-393.51kJ/mol
(298.15K)=-393.51kJ/mol
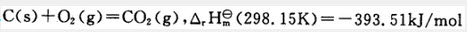


 2C(s)+2O2(g)=2CO2(g),
2C(s)+2O2(g)=2CO2(g), C+O2=CO2,
C+O2=CO2,

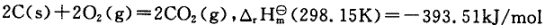 C(s)+O
C(s)+O (g)=COz(g)
(g)=COz(g) 