你可能感兴趣的试题
ΔH1>0,ΔH3<0 ΔH2>0,ΔH4>0 ΔH1=ΔH2+ΔH3 ΔH3=ΔH4+ΔH5
2H2S + SO2 = 3S↓ + 2H2O (复分解反应) CO2 +  2CO (化合反应) C.2AgBr
2CO (化合反应) C.2AgBr 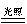 2Ag + Br2↑ (分解反应) 2Mg + CO2
2Ag + Br2↑ (分解反应) 2Mg + CO2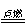 2MgO +C. (置换反应)
2MgO +C. (置换反应)
稀硫酸滴在铜片上:Cu + 2H+ = Cu2+ + H2O 氧化镁与稀盐酸混合:MgO +2H+ = Mg2+ + H2O 铜片插入硝酸银溶液中:Cu +Ag+ = Cu2+ + Ag 稀硝酸滴在石灰石上:Ca CO3+2H+ = Ca2+ + H2 CO3
Zn+2HCl═ZnCl2+H2↑ 2C+O2  2CO C.+CO2
2CO C.+CO2  2CO 2CO+O2
2CO 2CO+O2  2CO2
2CO2
Zn(s)+Ag2O(s)=ZnO(s)+2Ag(s) ΔH.=+317.3kJ·mol-1 Zn+Ag2O=ZnO+2Ag ΔH.=+317.3kJ·mol-1 Zn(s)+Ag2O(s)=ZnO(s)+2Ag(s) ΔH.=-317.3kJ 2Zn(s)+2Ag2O(s)=2ZnO(s)+4Ag(s) ΔH.=-634.6 kJ·mol-1
2ΔH1=ΔH2 ΔH1<0,ΔH2>0 ΔH5=ΔH3+2ΔH1-ΔH2 2ΔH1 -ΔH2>0
2C O (g)+ O2(g)==2CO2(g) HNO3(aq)+NaOH(aq)==NaNO3(aq)+H2O(l) 2H2(g)+O2(g)==2H2O(l) Zn(s)+ Ag2 O(s)== Zn O(s)+2Ag(s)
2H2S + SO2 = 3S↓ + 2H2O (复分解反应) CO2 +  2CO (化合反应) C. 2AgBr
2CO (化合反应) C. 2AgBr 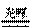 2Ag + Br2↑(分解反应) 2Mg + CO2
2Ag + Br2↑(分解反应) 2Mg + CO2  2MgO +C.(置换反应)
2MgO +C.(置换反应)
Zn(s)十Ag2O(s)====ZnO(s)+2Ag(s),△H=317.3kJ/mol Zn+Ag2O=====ZnO+2Ag,△H=317.3kJ/mol Zn(s)+Ag2O(s)=====ZnO(s)+2Ag(s),△H=-317.3kJ/mol 2Zn(s)+2Ag2O(s)=====2ZnO(s)+4Ag(s),△H=-317.3kJ
S(s) + O2 (g) =SO2 (g); △H1 S(g) + O2 (g) =SO2 (g);△H2 2H2(g) + O2 (g)= 2H2O(g);△H1 2H2 (g) + O2 (g) = 2H2O(l);△H2 CO(g) + 1/2 O2(g) = CO2(g);△H1 2CO(g) + O2(g) = 2CO2(g);△H2 H2 (g) +Cl2(g)=2HCl(g);△H1 1/2 H2(g) + 1/2 Cl2(g) = HCl(g);△H2
2H2S + SO2 = 3S↓ + 2H.2O. (复分解反应) CO2 +  2CO (化合反应) C.2AgBr
2CO (化合反应) C.2AgBr  2Ag + Br2↑ (分解反应) 2Mg + CO2
2Ag + Br2↑ (分解反应) 2Mg + CO2 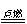 2MgO +C. (置换反应)
2MgO +C. (置换反应)
2H2(g) + O2(g)= 2H2O(g) △H1 ; 2H2(g) + O2(g) = 2H2O(l) △H2 S(g) + O2(g) =SO2(g) △H1 ; S(s) + O2(g) = SO2(g) △H2 CO(g) + 1/2 O2(g) = CO2(g) △H1 ;2CO(g) + O2(g) = 2CO2(g) △H2 C.(s)+H2O(g)=CO(g)+H2(g) △H1 ;CaO(s)+H2O(l)===Ca(OH)2(s) △H2
2H2S + SO2 = 3S↓ + 2H2O (复分解反应) 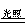 CO2 + 2CO (化合反应)
CO2 + 2CO (化合反应) 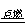 C.2AgBr 2Ag + Br2↑ (分解反应) 2Mg + CO2 2MgO +C. (置换反应)
C.2AgBr 2Ag + Br2↑ (分解反应) 2Mg + CO2 2MgO +C. (置换反应)
Zn(s)+Ag2O(s)===ZnO(s)+2Ag(s)ΔH.=+317.3kJ·mol-1 Zn+Ag2O===ZnO+2Ag ΔH.=+317.3kJ·mol-1 Zn(s)+Ag2O(s)===ZnO(s)+2Ag(s)ΔH.=-317.3kJ 2Zn(s)+2Ag2O(s)===2ZnO(s)+4Ag(s)ΔH.=-634.6 kJ·mol-1
2Fe+6HCl===2FeCl3+3H2↑ C.+2CuO===CO2↑+2Cu Cu+2AgNO3===Cu(NO3)2+2Ag Mg+O2===MgO2

 2CO (化合反应) C.2AgBr
2CO (化合反应) C.2AgBr 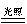 2Ag + Br2↑ (分解反应) 2Mg + CO2
2Ag + Br2↑ (分解反应) 2Mg + CO2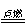 2MgO +C. (置换反应)
2MgO +C. (置换反应)
 2CO C.+CO2
2CO C.+CO2  2CO 2CO+O2
2CO 2CO+O2  2CO2
2CO2
 2CO (化合反应) C. 2AgBr
2CO (化合反应) C. 2AgBr 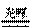 2Ag + Br2↑(分解反应) 2Mg + CO2
2Ag + Br2↑(分解反应) 2Mg + CO2  2MgO +C.(置换反应)
2MgO +C.(置换反应)
 2CO (化合反应) C.2AgBr
2CO (化合反应) C.2AgBr  2Ag + Br2↑ (分解反应) 2Mg + CO2
2Ag + Br2↑ (分解反应) 2Mg + CO2 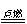 2MgO +C. (置换反应)
2MgO +C. (置换反应)
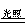 CO2 + 2CO (化合反应)
CO2 + 2CO (化合反应) 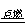 C.2AgBr 2Ag + Br2↑ (分解反应) 2Mg + CO2 2MgO +C. (置换反应)
C.2AgBr 2Ag + Br2↑ (分解反应) 2Mg + CO2 2MgO +C. (置换反应)