你可能感兴趣的试题
C=k0(1-e-kt)/Vk logC’=(-k/2.303)t’+log(k0/Vk) logC’=(-k/2.303)t’+log(k0(1-e-kt)/Vk logC=(-k/2.303)t+logC0 logX=(-k/2.303)t+logX0
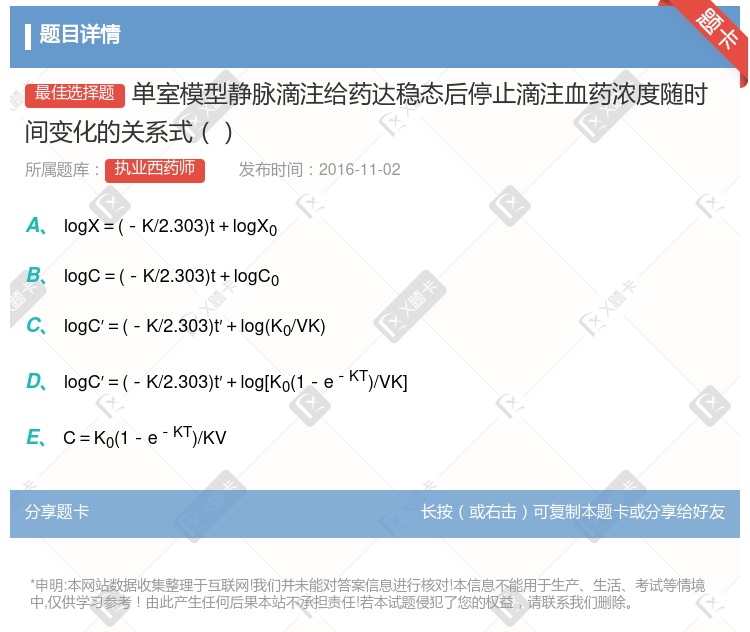
logX=(-K/2.303)t+logX0 logC=(-K/2.303)t+logC0 logC′=(-K/2.303)t′+log(K0/VK) logC′=(-K/2.303)t′+log[K0(1-e-KT)/VK] C=K0(1-e-KT)/KV
1个半衰期 2个半衰期 3个半衰期 4个半衰期 5个半衰期
C=C0(1-e-kt)/Vk logC’=(-k/2.303)t’+log(k0/Vk) logC’=(一k/2.303)t’+log(k0(1-e-kt)/Vk logC=(一k/2.303)t+logC0 logX=(一k/2.303)t+logX0
单室模型静脉注射给药,1gC对t作图,得到直线的斜率为负值 单室模型静脉滴注给药,在滴注开始时可以静注一个负荷剂量,使血药浓度迅速达到或接近稳态浓度 单室模型口服给药,在血药浓度达峰瞬间,吸收速度等于消除速度 多剂量给药、血药浓度波动与药物半衰期,给药间隔时间有关 多剂量给药、相同给药间隔下、半衰期短的药物容易蓄积
1个半衰期 2个半衰期 3个半衰期 4个半衰期 5个半衰期
logX=(-K/2.303)t+logX0 logC=(-K/2.303)t+logC0 logC′=(-K/2.303)t′+log(K0/VK) logC′=(-K/2.303)t′+log[K0(1-e-KT)/VK] C=K0(1-e-KT)/KV
C=C0(1-e-kt)/Vk logC’=(-k/2.303)t’+log(k0/Vk) logC’=(一k/2.303)t’+log(k0(1-e-kt)/Vk logC=(一k/2.303)t+logC0 logX=(一k/2.303)t+logX0
logX=(-K/2.303)t+logX0 10gC=(-K/2.303)t+logC0 logC′=(-K/2.303)t′+log(K0/VK) 10gC′=(-K/2.303)t′+log[K0(1-e-Kt)/VK] C=K0(1-e-Kt)/KV
logX=(-K/2.303)t+logX0 logC=(-K/2.303)t+logC0 logC′=(-K/2.303)t′+log(K0/VK) logC′=(-K/2.303)t′+log[K0(1-e-KT)/VK] C=K0(1-e-KT)/KV
logX=(-K/2.303)t+logX0 10gC=(-K/2.303)t+logC0 logC′=(-K/2.303)t′+log(K0/VK) 10gC′=(-K/2.303)t′+log[K0(1-e-KT)/VK] C=K0(1-e-Kt)/KV
logX=(-K/2.303)t+logX0 10gC=(-K/2.303)t+logC0 logC′=(-K/2.303)t′+log(K0/VK) 10gC′=(-K/2.303)t′+log[K0(1-e-KT)/VK] C=K0(1-e-Kt)/KV
血药浓度达稳态后,加快滴注速率,血药浓度又会重新上升并趋于稳定 滴注开始时,药物的消除速率大于给药速率 滴注速率越大,达到稳态血药浓度的时间越短 达到稳态血药浓度的75%所需要的滴注时间是3个生物半衰期 可通过减漫滴注速率的方式降低药物的消除速率
C=K0(1-e-kt)/VK logC’=(-K/2.303)t’+log(K0/VK) logC’=(-K/2.303)t’+log(K0(1-e-kt)/VK) logC=(-K/2.303)t+logC0 logX=(-K/2.303)t+logX0
C=K0(1-e-kt)/VK logC’=(-K/2.303)t’+log(K0/VK) logC’=(-K/2.303)t’+log(K0(1-e-KT)/VK) logC=(-K/2.303)t+logC0 logX=(-K/2.303)t+logX0