你可能感兴趣的试题
H2O(g)=H2(g)+1/2O2(g) △H.(298K)=+242KJ/mol 2H2(g)+O2(g)=2H2O(1) △H.(298K)=-484KJ/mol H2(g)+1/2O2(g)=H2O(g) △H.(298K)=+242KJ/mol 2H2(g)+O2(g)=2H2O(g) △H.(298K)=+484KJ/mol
H2O(g) === H2(g)+错误!未找到引用源。O2(g) △H=+242kJ•mol-1 2H2(g)+O2(g) === 2H2O(l) △H=-484kJ•mol-1 H2(g)+ 错误!未找到引用源。O2(g) === 2H2O(g) △H=+242kJ•mol-1 2H2(g)+O2(g) === 2H2O(g) △H=+484kJ•mol-1
每2个氢分子和1个氧分子,在点燃的条件下反应生成2个水分子 氢气和氧气在点燃的条件下反应生成水 氢元素和氧元素在点燃的条件下反应生成水 每2mol氢气跟1mol氧气在点燃的条件下反应生成2mol水
Sn+2Cl2=SnCl4 ΔH(298K)=-154.4kJ・mol-1
Sn(s)+2Cl2(g)=SnCl4(s) ΔH(298K)=-545.2kJ
Sn(s)+2Cl2(g)=SnCl4(s) ΔH(298K)=-154.4kJ・mol-1
Sn(s)+2Cl2(g)=SnCl4(s) ΔH(298K)=-545.2kJ・mol-1
H2O(g)=H2(g)+1/2 O2(g) ΔH=+242 kJ/mol 2 H2(g)+O2(g)=2 H2O(g) ΔH=-484 kJ/mol H2(g)+1/2 O2(g)=H2O(g) ΔH=+242 kJ/mol 2 H2(g)+O2(g)=2 H2O(g) ΔH=+484 kJ/mol
1分子H2和Cl2反应,放出热量184.6kJ 在101kPa,25oC的条件下,1mol H2(g)和1mol Cl2(g)完全反应生成2mol HCl(g),放出的热量为184.6kJ 1mol H2(g)完全反应生成2mol HCl(g),放出的热量为184.6kJ 在101kPa,25oC的条件下,1mol H2和1mol Cl2完全反应生成2mol HCl(g),吸收的热量为184.6kJ
2mol水蒸气分解成2mol氢气与1mol氧气吸收270kJ热量
2mol氢气与1mol氧气反应生成2mol液态水放出热量大于270kJ
在相同条件下,2mol氢气与1mol氧气的能量总和大于2mol水蒸汽的能量
2个氢气分子与1个氧气分子反应生成2个水蒸汽分子放出270kJ热量
H2O(g)=H2(g)+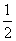 O2(g);
O2(g); H.= -242kJ·mol-1 2H2(g)+O2(g)=2H2O(l);
H.= -242kJ·mol-1 2H2(g)+O2(g)=2H2O(l); H.= -484kJ·mol-1 H2(g)+
H.= -484kJ·mol-1 H2(g)+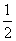 O2(g)=H2O(g);
O2(g)=H2O(g); H.= +242kJ·mol-1 2H2(g)+O2(g)=2H2O(g);
H.= +242kJ·mol-1 2H2(g)+O2(g)=2H2O(g); H.= -484kJ·mol-1
H.= -484kJ·mol-1
H2O(g) === H2(g)+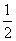 O2(g) △H.= +242kJ·mol-1 2H2(g)+O2(g)===2H2O(l) △H.= -484kJ·mol-1 H2(g)+
O2(g) △H.= +242kJ·mol-1 2H2(g)+O2(g)===2H2O(l) △H.= -484kJ·mol-1 H2(g)+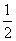 O2(g)===H2O(g) △H.= +242kJ·mol-1 2H2(g)+O2(g)===2H2O(g) △H.= +484kJ·mol-1
O2(g)===H2O(g) △H.= +242kJ·mol-1 2H2(g)+O2(g)===2H2O(g) △H.= +484kJ·mol-1
H20(g)= H2(g)+ 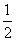 O2(g);△H=+242kJ・mol-1 2H2(g)+02(g)= 2H2O(1);△H= -484 kJ・mol-1 H2(g)+
O2(g);△H=+242kJ・mol-1 2H2(g)+02(g)= 2H2O(1);△H= -484 kJ・mol-1 H2(g)+ 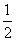 O2(g)=H2O(g);△H=+242 kJ・mol-1 2H2(g)+02(g)= 2H2O(g);△H= -484 kJ・mol-1
O2(g)=H2O(g);△H=+242 kJ・mol-1 2H2(g)+02(g)= 2H2O(g);△H= -484 kJ・mol-1
H2O(g) = H2(g) + 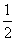 O2 (g) △H.= +242 kJ/mol 2H2(g) + O2 (g) = 2H2O (l) △H.= -484 kJ/mol H2 (g) +
O2 (g) △H.= +242 kJ/mol 2H2(g) + O2 (g) = 2H2O (l) △H.= -484 kJ/mol H2 (g) + 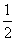 O2 (g) = H2O (l) △H.= -242 kJ/mol 2H2 (g) + O2 (g) = 2H2O (g) △H.= +484 kJ/mol
O2 (g) = H2O (l) △H.= -242 kJ/mol 2H2 (g) + O2 (g) = 2H2O (g) △H.= +484 kJ/mol
H2O(g)=H2(g)+1/2O2(g) ΔH.=+242kJ·mol-1 2H2(g)+O2(g)=2H2O(l) ΔH.=-484kJ·mol-1 H2(g)+1/2O2(g)=H2O(g) ΔH.=+242kJ·mol-1 2H2(g)+O2(g)=2H2O(g) ΔH.=+484kJ·mol-1
H2O(g) === H2(g)+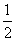 O2(g) △H.= +242kJ·mol-1 2H2(g)+O2(g)===2H2O(l) △H.= -484kJ·mol-1 H2(g)+
O2(g) △H.= +242kJ·mol-1 2H2(g)+O2(g)===2H2O(l) △H.= -484kJ·mol-1 H2(g)+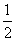 O2(g)===H2O(g) △H.= +242kJ·mol-1 2H2(g)+O2(g)===2H2O(g) △H.= +484kJ·mol-1
O2(g)===H2O(g) △H.= +242kJ·mol-1 2H2(g)+O2(g)===2H2O(g) △H.= +484kJ·mol-1
1676kJ -1676kJ
3352kJ -3352kJ
H2O(g)=H2(g)+1/2O2(g) ΔH.=+242kJ・mol-1 2H2(g)+O2(g)=2H2O(l) ΔH.=-484kJ・mol-1 H2(g)+1/2O2(g)=H2O(g) ΔH.=+242kJ・mol-1 2H2(g)+O2(g)=2H2O(g) ΔH.=+484kJ・mol-1
2H2(g)+O2(g)=2H2O(g);△H=+484kJ・mol-1 2H2(g)+O2(g)=2H2O(l);△H=-484kJ・mol-1 2H2(g)+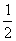 O2(g)=2H2O(g);△H=+242kJ・mol-1 2H2(g)=H2(g)+
O2(g)=2H2O(g);△H=+242kJ・mol-1 2H2(g)=H2(g)+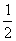 O2(g);△H=+242kJ・mol-1
O2(g);△H=+242kJ・mol-1
H2O(g)=H2(g)+ 1/2O2 (g)△H=+242 kJ.mol-1 2H2(g)+O2(g)=2H2O(l) △H= -484 kJ.mol-l H2(g)+ 1/2O2(g)=H2O(g) △H=+242 kJ.mol-l 2H2(g) + O2(g) = 2 H2O(g) △H= -484 kJ

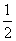 O2(g);
O2(g); H.= -242kJ·mol-1 2H2(g)+O2(g)=2H2O(l);
H.= -242kJ·mol-1 2H2(g)+O2(g)=2H2O(l); H.= -484kJ·mol-1 H2(g)+
H.= -484kJ·mol-1 H2(g)+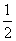 O2(g)=H2O(g);
O2(g)=H2O(g); H.= +242kJ·mol-1 2H2(g)+O2(g)=2H2O(g);
H.= +242kJ·mol-1 2H2(g)+O2(g)=2H2O(g); H.= -484kJ·mol-1
H.= -484kJ·mol-1
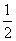 O2(g) △H.= +242kJ·mol-1 2H2(g)+O2(g)===2H2O(l) △H.= -484kJ·mol-1 H2(g)+
O2(g) △H.= +242kJ·mol-1 2H2(g)+O2(g)===2H2O(l) △H.= -484kJ·mol-1 H2(g)+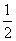 O2(g)===H2O(g) △H.= +242kJ·mol-1 2H2(g)+O2(g)===2H2O(g) △H.= +484kJ·mol-1
O2(g)===H2O(g) △H.= +242kJ·mol-1 2H2(g)+O2(g)===2H2O(g) △H.= +484kJ·mol-1
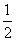 O2(g);△H=+242kJ・mol-1 2H2(g)+02(g)= 2H2O(1);△H= -484 kJ・mol-1 H2(g)+
O2(g);△H=+242kJ・mol-1 2H2(g)+02(g)= 2H2O(1);△H= -484 kJ・mol-1 H2(g)+ 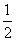 O2(g)=H2O(g);△H=+242 kJ・mol-1 2H2(g)+02(g)= 2H2O(g);△H= -484 kJ・mol-1
O2(g)=H2O(g);△H=+242 kJ・mol-1 2H2(g)+02(g)= 2H2O(g);△H= -484 kJ・mol-1
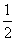 O2 (g) △H.= +242 kJ/mol 2H2(g) + O2 (g) = 2H2O (l) △H.= -484 kJ/mol H2 (g) +
O2 (g) △H.= +242 kJ/mol 2H2(g) + O2 (g) = 2H2O (l) △H.= -484 kJ/mol H2 (g) + 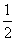 O2 (g) = H2O (l) △H.= -242 kJ/mol 2H2 (g) + O2 (g) = 2H2O (g) △H.= +484 kJ/mol
O2 (g) = H2O (l) △H.= -242 kJ/mol 2H2 (g) + O2 (g) = 2H2O (g) △H.= +484 kJ/mol
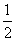 O2(g) △H.= +242kJ·mol-1 2H2(g)+O2(g)===2H2O(l) △H.= -484kJ·mol-1 H2(g)+
O2(g) △H.= +242kJ·mol-1 2H2(g)+O2(g)===2H2O(l) △H.= -484kJ·mol-1 H2(g)+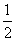 O2(g)===H2O(g) △H.= +242kJ·mol-1 2H2(g)+O2(g)===2H2O(g) △H.= +484kJ·mol-1
O2(g)===H2O(g) △H.= +242kJ·mol-1 2H2(g)+O2(g)===2H2O(g) △H.= +484kJ·mol-1
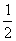 O2(g)=2H2O(g);△H=+242kJ・mol-1 2H2(g)=H2(g)+
O2(g)=2H2O(g);△H=+242kJ・mol-1 2H2(g)=H2(g)+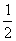 O2(g);△H=+242kJ・mol-1
O2(g);△H=+242kJ・mol-1