
 2NH3(l) ΔH.=2(a-b-c) kJ·mol-1 N2(g)+3H2(g)
2NH3(l) ΔH.=2(a-b-c) kJ·mol-1 N2(g)+3H2(g) 2NH3(g) ΔH.=2(b-a) kJ·mol-1 1/2N2(g)+3/2H2(g)
2NH3(g) ΔH.=2(b-a) kJ·mol-1 1/2N2(g)+3/2H2(g) NH3(l) ΔH.=(b+c-a) kJ·mol-1 1/2N2(g)+3/2H2(g)
NH3(l) ΔH.=(b+c-a) kJ·mol-1 1/2N2(g)+3/2H2(g) NH3(g) ΔH.=(a+b) kJ·mol-1
NH3(g) ΔH.=(a+b) kJ·mol-1
 N2(g)+
N2(g)+ H2(g) = NH3(l) ;△H.= (b+c—a) kJ/mol
H2(g) = NH3(l) ;△H.= (b+c—a) kJ/mol  N2(g)+
N2(g)+ H2(g) =NH3(g) ;△H.= (a+b) kJ/mol
H2(g) =NH3(g) ;△H.= (a+b) kJ/mol
 2NH3(l) ΔH.=-2(b+c - a)kJ/mol N2(g)+3H2(g)
2NH3(l) ΔH.=-2(b+c - a)kJ/mol N2(g)+3H2(g) 2NH3(g) ΔH. =-2(a - b)kJ/mol N2(g)+3H2(g)
2NH3(g) ΔH. =-2(a - b)kJ/mol N2(g)+3H2(g)  2NH3(l) ΔH.=+(b+c - a)kJ/mol N2(g)+3H2(g)
2NH3(l) ΔH.=+(b+c - a)kJ/mol N2(g)+3H2(g)  2NH3(g) ΔH.=+(a+b)kJ/mol
2NH3(g) ΔH.=+(a+b)kJ/mol
 2NH3g) △H =-38.6kJ/mol
2NH3g) △H =-38.6kJ/mol
 N2(g)+
N2(g)+ H2(g) = NH3(l) ;⊿H = (b+c—a)kJ·mol-1
H2(g) = NH3(l) ;⊿H = (b+c—a)kJ·mol-1  N2(g)+
N2(g)+ H2(g) =NH3(g) ;⊿H = (a+b)kJ·mol-1
H2(g) =NH3(g) ;⊿H = (a+b)kJ·mol-1
 N2(g)+
N2(g)+ H2((g)=NH3(g);ΔH.=(a+b) kJ·mol-1 N2(g)+3H2(g)=2NH3(g);ΔH.=2(b-a) kJ·mol-1
H2((g)=NH3(g);ΔH.=(a+b) kJ·mol-1 N2(g)+3H2(g)=2NH3(g);ΔH.=2(b-a) kJ·mol-1  N2(g)+
N2(g)+ H2(g)=NH3(l);ΔH.=(b+c-a) kJ·mol-1 N2(g)+3H2(g)=2NH3(l);ΔH.=2(a-b-c) kJ·mol-1
H2(g)=NH3(l);ΔH.=(b+c-a) kJ·mol-1 N2(g)+3H2(g)=2NH3(l);ΔH.=2(a-b-c) kJ·mol-1
 2NH3(1);△H=2(a-b-c)kJ·mol-1 N2(g)+3H2(g)
2NH3(1);△H=2(a-b-c)kJ·mol-1 N2(g)+3H2(g)  2NH3(g);△H=2(b-a)kJ·mol-1
2NH3(g);△H=2(b-a)kJ·mol-1  N2(g)+
N2(g)+ H2(g)
H2(g)  NH3(1);△H=(b+c-a)kJ·mol-1
NH3(1);△H=(b+c-a)kJ·mol-1  N2(g)+
N2(g)+ H2(g)
H2(g)  NH3(g);△H=(a+b)kJ·mol-1
NH3(g);△H=(a+b)kJ·mol-1
 2NH3(l) ⊿H = 2(a—b—c)kJ/mol N2(g)+3H2(g)
2NH3(l) ⊿H = 2(a—b—c)kJ/mol N2(g)+3H2(g)  2NH3(g) ⊿H = 2(b—a)kJ/mol 0.5N2(g)+1.5H2(g)
2NH3(g) ⊿H = 2(b—a)kJ/mol 0.5N2(g)+1.5H2(g)  NH3(l) ⊿H = (b+c—a)kJ/mol 0.5N2(g)+1.5H2(g)
NH3(l) ⊿H = (b+c—a)kJ/mol 0.5N2(g)+1.5H2(g)  NH3(g) ⊿H = (a+b)kJ/mol
NH3(g) ⊿H = (a+b)kJ/mol
 N2(g)+
N2(g)+ H2(g) = NH3(l) ;⊿H.= (b+c—a)kJ/mol
H2(g) = NH3(l) ;⊿H.= (b+c—a)kJ/mol  N2(g)+
N2(g)+ H2(g) =NH3(g) ;⊿H.= (a+b)kJ/mol
H2(g) =NH3(g) ;⊿H.= (a+b)kJ/mol
 2NH3(l) ⊿H = 2(a—b—c)kJ/mol N2(g)+3H2(g)
2NH3(l) ⊿H = 2(a—b—c)kJ/mol N2(g)+3H2(g)  2NH3(g) ⊿H = 2(b—a)kJ/mol 0.5N2(g)+1.5H2(g)
2NH3(g) ⊿H = 2(b—a)kJ/mol 0.5N2(g)+1.5H2(g)  NH3(l) ⊿H = (b+c—a)kJ/mol 0.5N2(g)+1.5H2(g)
NH3(l) ⊿H = (b+c—a)kJ/mol 0.5N2(g)+1.5H2(g)  NH3(g) ⊿H = (a+b)kJ/mol
NH3(g) ⊿H = (a+b)kJ/mol 
 N2(g)+
N2(g)+ H2(g) = NH3(l) ; ⊿H = (b+c—a)kJ·mol-1
H2(g) = NH3(l) ; ⊿H = (b+c—a)kJ·mol-1  N2(g)+
N2(g)+ H2 (g) =NH3(g) ; ⊿H = (a+b)kJ·mol-1
H2 (g) =NH3(g) ; ⊿H = (a+b)kJ·mol-1
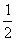 N2(g)+
N2(g)+ H2(g) = NH3(l) ;⊿H = (b+c—a)kJ·mol-1
H2(g) = NH3(l) ;⊿H = (b+c—a)kJ·mol-1 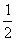 N2(g)+
N2(g)+ H2(g) =NH3(g) ;⊿H = (a+b)kJ·mol-1
H2(g) =NH3(g) ;⊿H = (a+b)kJ·mol-1

 2NH3(l) ΔH=2(a-b-c) kJ·mol-1 N2(g)+3H2(g)
2NH3(l) ΔH=2(a-b-c) kJ·mol-1 N2(g)+3H2(g)  2NH3(g) ΔH=2(b-a) kJ·mol-1
2NH3(g) ΔH=2(b-a) kJ·mol-1  N2(g)+
N2(g)+ H2(g)
H2(g)  NH3(l) ΔH=(b+c-a) kJ·mol-1
NH3(l) ΔH=(b+c-a) kJ·mol-1  N2(g)+
N2(g)+ H2(g)
H2(g)  NH3(g) ΔH=(a+b) kJ·mol-1
NH3(g) ΔH=(a+b) kJ·mol-1